Inhibiting mTORC1 Can Slow The Consequences of Muscle Aging
-
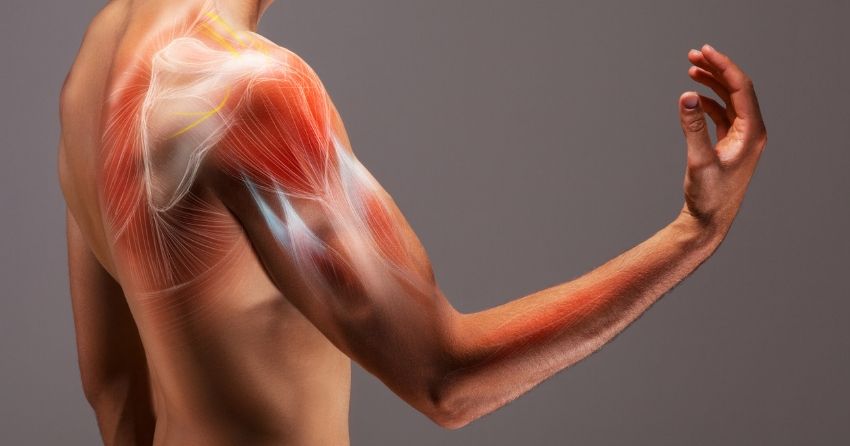
mTOR is a protein kinase that regulates cell growth, cell death, autophagy, and protein synthesis.
-
Inhibiting mTOR pathways has been shown to slow aging in mouse models.
-
Rapamycin has been found to inhibit mTORC1 and protect some organs against age-related decline, but its effect on skeletal muscle has not yet been studied.
This article was first published by Reason on FightAging.org
Inhibition of mTOR, and more specifically only its activities as a part of the mTORC1 protein complex, has been shown to slow aging in mice. This is a class of calorie restriction mimetic treatment, in that it works through many of the same beneficial stress response mechanisms as does a restricted nutrient intake. Many of the specific effects of mTORC1 inhibition in various different tissues in the body are still incompletely investigated, however. Researchers here discuss the effects of mTORC1 inhibition on the aging of muscle tissue. With age, muscle mass and strength are lost, leading to the onset of sarcopenia and contributing greatly to the condition of age-related frailty. Means to prevent this deterioration of muscle would provide a great benefit to the older population, but mTORC1 inhibition is unfortunately only a small step towards that goal.
This research was first published in Aging in 2019.
A decade ago, rapamycin, an mTORC1 inhibitor, was reported to extend the lifespan of mice. Consistent with this, rapamycin and other mTORC1 inhibitors also protect several organs and tissues against age-related functional decline. Rapamycin's effect on aging skeletal muscle, however, was not explored until recently. It has long been known that mTORC1 activity is induced in aging muscle. Two recent reports with genetic and pharmacological evidence reveal important findings: 1) Chronic activation of mTORC1 stimulates progressive muscle damage and loss, and 2) Inhibition of mTORC1 with rapamycin prevents age-related muscle loss.
Consistent with the observation that the hyperactive mTORC1 induces muscle damage and loss, inhibition of mTORC1 activity with rapamycin or rapalogs protects aging muscle from atrophy in mice and rats. For example, treatment with rapamycin from 9 months to 30 months of age reduced apoptosis and promoted retention of peripherally located nuclei, and this was associated with reduced fiber loss in aging skeletal muscle. In a separate study, a shorter duration of rapalog treatment for 6-weeks, starting from 22 month of age, preserved both fiber size and muscle weight. These data suggest that mTORC1 is necessary and sufficient to drive skeletal muscle aging.
Do these findings conflict with the current understanding of the anabolic function of mTORC1? We do not think so. We believe the ultimate effect of mTORC1 depends upon: 1) how mTORC1 is activated, and 2) for how long mTORC1 remains activated. Akt-dependent, short-term activation of mTORC1 appears usually to be beneficial to cells, whereas Akt-independent, chronic activation of mTORC1 appears to be detrimental to cells. Akt-independent activation of mTORC1 induces catabolism in addition to its conventional anabolic activity. The progressive myopathy seen in the mTORC1-hyperactive muscle is the net outcome of a complex process that perturbs the balance between anabolism and catabolism, with catabolism winning out and leading to muscle fiber damage and loss.







